NCL testified before CDC committee on COVID-19 vaccine recommendations
For immediate release: December 22, 2020
Media contact: National Consumers League – Carol McKay, carolm@nclnet.org, (412) 945-3242 or Taun Sterling, tauns@nclnet.org, (202) 207-2832
Washington, DC – The National Consumers League (NCL) testified before the Centers for Disease Control and Disease Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) this weekend on the equitable distribution of the Moderna vaccine and recommendations for consumer education on vaccine safety. In its testimony, NCL applauds the transparency and access afforded to the public throughout the COVID-19 vaccine approval process.
Equitable distribution:
NCL is encouraged that the Food and Drug Administration (FDA) has approved the Moderna vaccine and that the U.S. government will lead distribution efforts. Due to its ease of transport and storage, the Moderna vaccine stands to readily ship to rural and hard to reach communities. NCL calls on federal health officials at the helm of distribution to facilitate access to the Moderna vaccine to medically underserved areas.
Safety and efficacy:
NCL expressed its trust in the FDA and CDC’s robust inter-agency collaboration to continue ongoing, post-market surveillance of adverse events among recipients of the COVID-19 vaccine and to inform consumers of any additional safety recommendations. NCL urged the CDC to educate consumers about potential reactions and side effects, as this transparency will further encourage the compliance necessary to achieve herd immunity. The vaccine is expected to induce flu-like symptoms after the initial dose and this may deter some patients from getting their second dose if they aren’t warned about what to anticipate.
Vaccine adherence:
NCL encouraged the CDC to conduct culturally competent and inclusive public messaging about vaccine safety to ensure that communities of color and persons with limited English proficiency are informed and feel empowered in their decisions to vaccinate. Adding to the complexity of administering the vaccine, public health officials will need to ensure the completion of two doses in a series. This stands to create additional challenges because evidence has shown that when a vaccine involves multiple doses, nearly 50 percent of patients fail to return for a second dose.
Equitable allocation:
NCL applauds ACIP’s recommendations to prioritize vaccinations for health care workers and long-term care facility residents in Phase 1a. Now that there are two approved vaccines, NCL calls on ACIP to prioritize recommendations to vaccinate the approximately 87 million non-healthcare essential workers unable to work from home—such as bus drivers and grocery workers—who are at higher risk of exposure. Racial and ethnic minorities make up more than 40 percent of the essential workforce and are the backbone of many essential industries. The pandemic has illustrated that low-income minority communities experience more severe COVID-related illness requiring hospitalization and are at higher risk for death.
Persons who recovered from COVID-19:
Lastly, over 18 million individuals in the U.S. have been infected with the coronavirus. It is expected that individuals who recover will acquire some natural immunity to COVID-19. Individuals who recover from the coronavirus want to know if they are protected from reinfection and for how long. We call on the CDC to expedite developing vaccine recommendations for persons who’ve recovered from COVID-19.
###
About the National Consumers League (NCL)
The National Consumers League, founded in 1899, is America’s pioneer consumer organization. Our mission is to protect and promote social and economic justice for consumers and workers in the United States and abroad. For more information, visit www.nclnet.org.





 By NCL Director of Health Policy Jeanette Contreras
By NCL Director of Health Policy Jeanette Contreras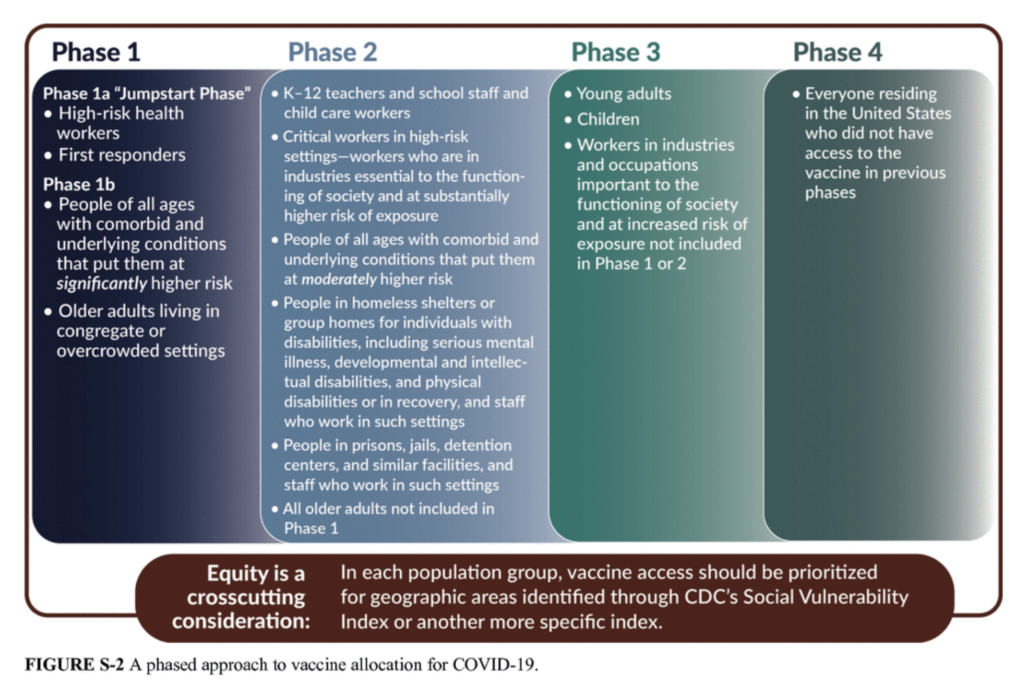

 Hispanic Heritage Month: Focus on the importance of participating in research through clinical trials
Hispanic Heritage Month: Focus on the importance of participating in research through clinical trials









