 Hispanic Heritage Month: Focus on the importance of participating in research through clinical trials
Hispanic Heritage Month: Focus on the importance of participating in research through clinical trials
by Elena Rios, MD, MSPH, FACP
President & CEO, National Hispanic Medical Association
The COVID-19 pandemic has impacted the world and the United States with a double threat: decreasing health and function of many, especially older patients with underlying diseases (obesity, asthma, diabetes, hypertension, etc.) that decrease the body’s immune response to fight off the virus; and millions left jobless as businesses downsize or close. In the healthcare arena, scientists and physicians are learning about the disease and how to treat it: We now know to limit ventilators to avoid high air pressures that can hurt damaged lungs; to place infected patients on their stomachs to allow lungs to expand; to use dexamethasone to decrease inflammation; and to use new antiviral therapies like Remdesivir and monoclonal antibodies. While there is no vaccine to prevent COVID-19, vaccine developers, researchers, and manufacturers are expediting the development of one.
The National Institutes of Health (NIH) and several pharmaceutical companies are conducting research through clinical trials that have found potential vaccines to be safe. This summer they started to enroll people and closely follow them for any adverse effects. Historically, Hispanics, Blacks, and Native Americans have been underrepresented in clinical trial research for a variety of factors, chief among them, a distrust of research and the concept of fatalism (leaving life’s challenges in God’s hands). But it is crucially important to have diversity in clinical trials to have information on the vaccine impact for Hispanics, for example. I encourage all persons over the age of 18 to enroll in the important COVID-19 clinical trials — and recommend websites for two ongoing clinical trials: the CoronaVirusPreventionNetwork.org from the NIH and Moderna, and the CovidVaccineStudy1.com
from Pfizer Inc. Each site provides consumers with information on the locations and how to enroll.
The National Hispanic Medical Association (NHMA) was established in 1994 to represent trusted Hispanic physicians and to improve the health of Hispanics and underserved populations. Given that, by 2042, one out of four people living in our nation will be Latino, NHMA has joined as a partner to encourage the Latino community to join the NIH All of Us Research Program. In May 2018, the NIH opened national enrollment for the All of Us Research Program—a momentous effort to advance individualized prevention, treatment, and care for people of all backgrounds—in collaboration with NHMA and other national partners. People ages 18 and older who reside in the United States, regardless of health status, are eligible to enroll. The overall aim is to enroll 1 million or more volunteers and to oversample communities that have historically been underrepresented in research to make the program the largest, most diverse resource of its kind. Our participation will provide information on how to better develop health care prevention and treatment programs for generations to come.
Precision medicine is an emerging approach to disease treatment and prevention that considers differences in people’s lifestyles, environments and biological makeup, including genes. By partnering with 1 million diverse people who share information about themselves over a 10-year period, the All of Us Research Program will enable research to more precisely prevent and treat a variety of health conditions.
Participants can access their own health information, including genetics information, summary data about the entire participant community, and information about studies and findings, that come from All of Us. Participants are asked to share different types of health and lifestyle information, through online surveys and electronic health records (EHRs), which will continue to be collected over the course of the program. At different times over the coming months and years, some participants will be asked to visit a local partner site to provide blood and urine samples and to have basic physical measurements taken, such as height and weight, to ensure that the program gathers information fromall types of people. This program is especially focused on those who have been underrepresented in research, but not everyone will be asked to give physical measures and samples. In the future, participants may be invited to share data through wearable devices and to join follow-up research studies, including clinical trials.
In addition, data from the program will be broadly accessible for research purposes. Ultimately, the All of Us Research program will be a rich and open data resource for traditional academic researchers as well as citizen scientists—and everyone in between. To learn more about the program and how to join, please visit https://www.JoinAllofUs.org.
About NHMA
NHMA is a nonprofit association representing the interests of 50,000 Hispanic physicians with the mission to improve the health of Hispanics in the U.S. For more information, please visit www.NHMAmd.org


 By NCL Director of Health Policy Jeanette Contreras
By NCL Director of Health Policy Jeanette Contreras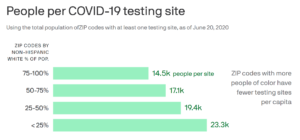


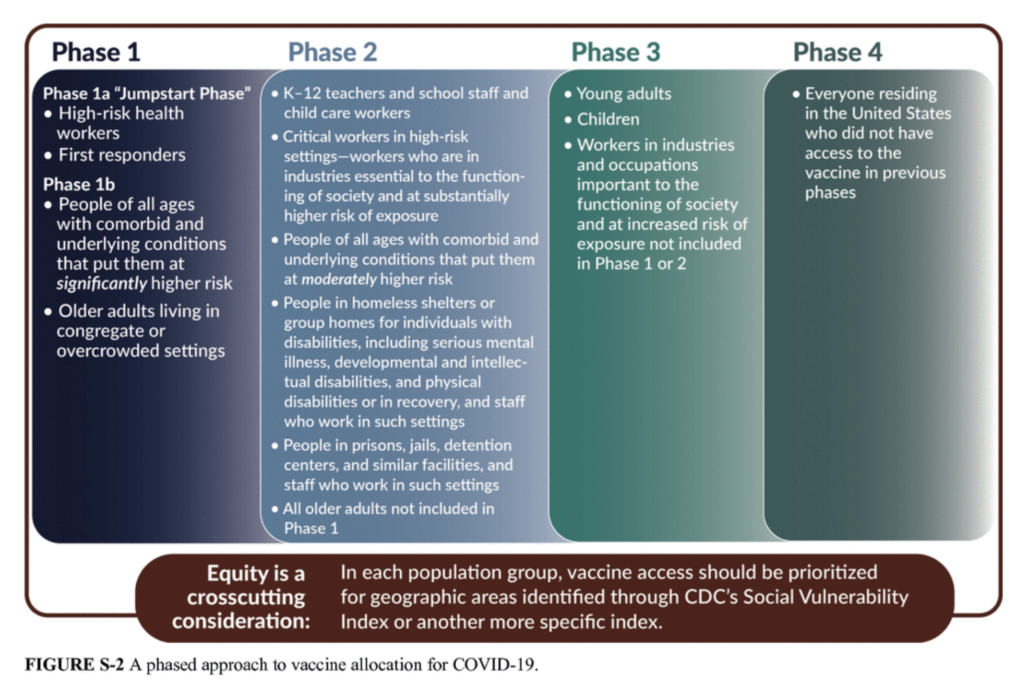

 Hispanic Heritage Month: Focus on the importance of participating in research through clinical trials
Hispanic Heritage Month: Focus on the importance of participating in research through clinical trials













