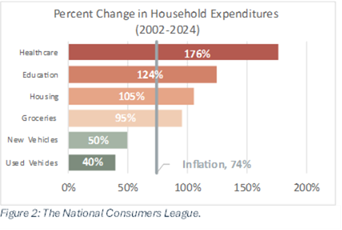NCL Applauds Subcommittee Passage of Safety is Not For Sale, PART Act
Media Contact: Lisa McDonald, Vice President of Communications, 202-207-2829
Washington, DC – The House Subcommittee on Commerce, Manufacturing, and Trade on Tuesday reported 12 bills to full committee that seek to improve roadway safety, strengthen American automobile manufacturing, and protect vehicle owners from unfair business practices. The National Consumers League (NCL) submitted a letter for the record, urging Congress to enact critical reforms to save lives, reduce injuries, support innovation, and spur economic growth.
“The death and destruction on our nation’s roads does not have to be the price we pay for commuting to work, dropping the kids off at school, or picking up groceries,” the letter states. “We applaud you for seeking to reduce the unacceptable loss of life, physical injuries, and economic costs attributable to motor vehicle crashes.”
“NCL strongly supports the Safety is Not for Sale Act, as such legislation is vital to ensuring consumers have more affordable access to life-saving automobile safety features,” the letter continues. “NCL found that some advanced driving assistance systems (ADAS) are sold as luxury items that must be purchased for an extra fee or as part of expensive add-on packages. These additional costs may put these life-saving technologies out of reach for many Americans.”
NCL also expressed support for the PART Act, which requires the National Highway Traffic Safety Administration (NHTSA) to mandate that catalytic converters be affixed with antitheft markings, establishes a grant program to facilitate the marking of catalytic converters currently deployed in interstate commerce, and increases criminal penalties for catalytic converter theft.
“We are encouraged that the Committee is taking action to prevent catalytic converter theft, which has become a major consumer and environmental protection issue,” the letter states.
A copy of the letter can be found HERE.
###
About the National Consumers League (NCL)
The National Consumers League, founded in 1899, is America’s pioneer consumer organization. Our mission is to protect and promote social and economic justice for consumers and workers in the United States and abroad. For more information, visit www.nclnet.org.









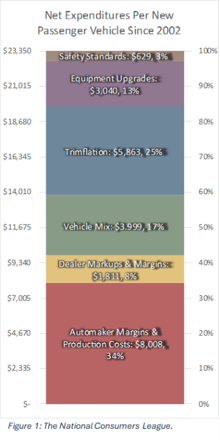 “Families don’t have to choose between safety, fuel efficiency, and vehicle affordability, and the data proves it,” said
“Families don’t have to choose between safety, fuel efficiency, and vehicle affordability, and the data proves it,” said