By Robin Strongin, Health Policy Director

Bill Tompkins
Here we go, again.
Up until the Dobbs decision in 2022, Roe v Wade had been the law of the land since 1973.
Up until today, women didn’t have to worry that mifepristone, approved by FDA in 2000, would be available as a safe and effective and legal way to end an early pregnancy.
But, Texas Federal Judge Matthew Kacsmaryk, who has been vehemently anti-choice his entire life, is the judge who will decide whether to issue a preliminary injunction ordering FDA to withdraw its longstanding approval of mifepristone, the first pill in the two-drug medication abortion regimen.
Women, and their health providers, stand at a crossroads. All women, not just those in Texas.
For some, it’s not enough that Roe was overturned in 2022. Back in November 2022 the Alliance Defending Freedom, a conservative legal group, filed a lawsuit on behalf of antiabortion medical organizations and doctors. At issue is the FDA’s approval of mifepristone, otherwise known as the medication abortion pill. The plaintiffs, led by the Alliance for Hippocratic Medicine, have asked the judge to issue a preliminary injunction ordering the FDA to withdraw mifepristone.
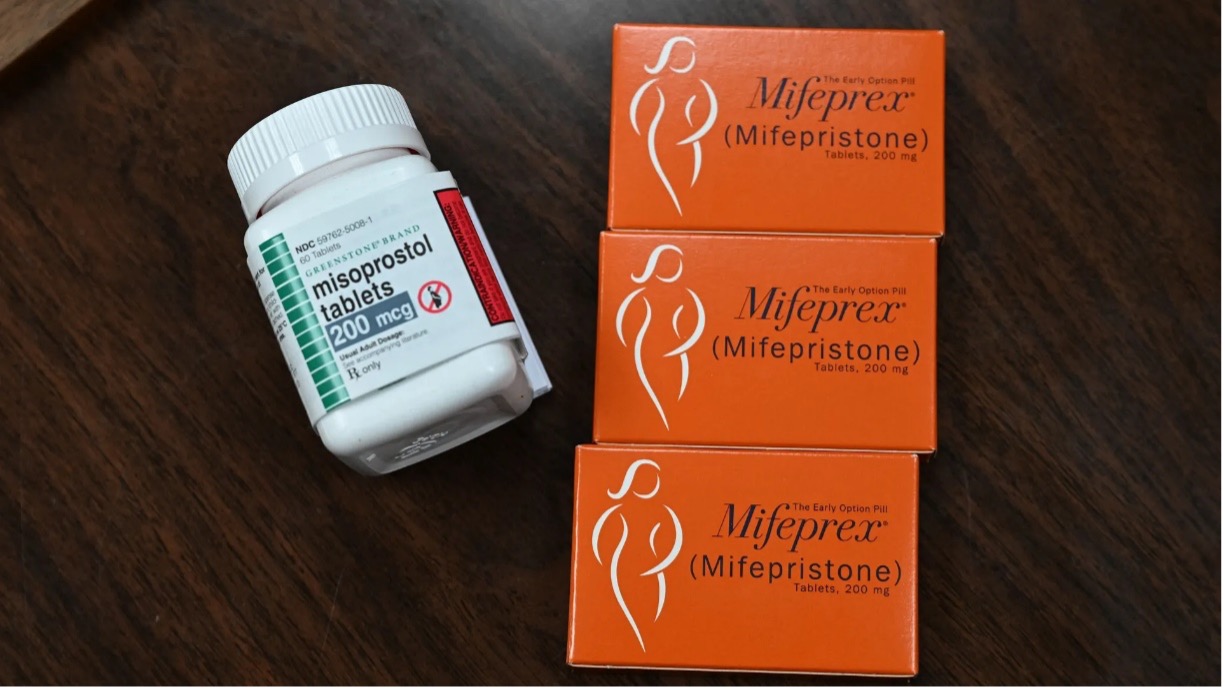
Photo: Robyn Beck / AFP via Getty Images
This is unchartered territory. The court is being asked, for the first time, to basically overturn the approval of a drug. A drug that has been safely used for decades by over 5.6 million[i] women. The drug regimen terminates pregnancies successfully 99.6% of the time, with a 0.4% risk of major complications, and an associated mortality rate of less than 0.001 percent (0.00064%).[ii]
This case, and several others that have been brought forward on medication abortion, raise questions about the role of the courts in reviewing the FDA’s findings about a particular drug. This is chilling. Not only for the women who rely on this medication, but this case has the potential to set up an extremely alarming precedent for other FDA approved drugs.
And it’s dangerous territory for women and their health providers. According to a court filing, FDA stated that overturning its approval of mifepristone would “cause significant harm, depriving patients of a safe and effective drug that has been on the market for more than two decades.”
Should Judge Kacsmaryk rule in favor of the plaintiffs, over half the abortions in the US could come to a halt—this includes abortions in states where abortion rights are (still) protected. This case is expected to find its way to the Supreme Court—to the same justices who overturned Roe v Wade.
Through a coordinated strategy to take away women’s reproductive rights, advancing abortion bans—at the federal level, at the state level, and through the courts, results in confusion, fear, and poor health outcomes.
Fortunately, experts seem to agree that if the worst were to happen, and the preliminary injunction is granted (and remain in place following the inevitable appeals), there are several options that could allow for the continuing supply of the drug and for providers to continue prescribing.
For example, some abortion providers are planning to provide only the second abortion medication, misoprostol, which is used safely on its own in many countries, though it does have more side effects than mifepristone.
Earlier this week, Governor Gavin Newsom (D-Calif) announced that California state government would no long do business with Walgreens because of their decision that it won’t sell mifepristone in states where Republicans have threatened legal action, even in those states where abortion remians legal.
All of this is happening during Women’s History Month. But, knowledge is power. According to the Guttmacher Institute, a leading research and policy organization committed to advancing sexual and reproductive health and rights worldwide, “Since its approval, medication abortion has been used over four million times and has become so widely accepted by patients and providers that it now accounts for more than half of all US abortions—492,210 of the 930,160 abortions (53%) provided in 2020 were done with abortion pills.”
According to the Guttmacher researchers, the impact of eliminating access to medication abortion would differ greatly state to state, but could be especially promounced in rural counties and regions of any state….These 10 states could experience the most severe impact:
Colorado, Georgia, Indiana, Iowa, Maine, Montana, New Mexico, Pennsylvania,Vermontand Washington.
Guttmacher created an interactive map, capturing abortion-related policies and data, categorizing states from the most restrictive to the most protective.
On the map, viewers can also see demographic information and key abortion statistics. The data for women of reproductive age* in each state include:
- Age-groups and race/ethnicity
- Proportion living below 200% of the federal poverty level
- Types of health insurance used
- Proportion born outside the United State
Abortion-related statistics for each state include:
- Number and rate of abortions provided
- Number of clinics that provide abortions
- Average driving distance to the nearest abortion clinic
The new interactive map is available here.
*The use of “women” to refer to the population of people potentially impacted by abortion policies reflects the terminology in the US census, from which many of our data points are drawn. We recognize that gender identities are diverse and not everyone who needs an abortion may identify as a woman. We reflect that reality in our language where we can, while also accurately describing the underlying data.
[i] Mifepristone US Post-Marketing Adverse Events Summary through 6/30/2022; TTT #2022-2468. NDA 020687. ANDA 091178. www.fda.gov
[ii] Mifepristone US Post-Marketing Adverse Events Summary through 12/31/2018; RCM #2007-525. NDA 20-687. www.fda.gov