Are the olives used to make your olive oil contaminated with herbicides?
 Shaunice Wall is NCL’s Linda Golodner Food Safety and Nutrition Fellow
Shaunice Wall is NCL’s Linda Golodner Food Safety and Nutrition Fellow
Olive oil and the Mediterranean are almost synonymous. Extra virgin olive oil, or EVOO, has been an unrivaled staple in the gastronomy of the health conscious. It has also been long time dubbed as “heart healthy” due to its high antioxidant and monosaturated fats, which can help lower “bad” LDL cholesterol and raise “good” HDL cholesterol. In fact, the Mediterranean diet was recommended as one of the healthiest diets in the latest edition of the Dietary Guidelines for Americans.
However, fraud in the marketing of EVOO has plagued consumers for decades. Too often, EVOO on the shelves of our grocery stores are low quality and falsely marked as high-quality virgin or extra-virgin olive oil. An additional common fraudulent activity of olive oil production is the mixing of fresh extra virgin olive oil with inferior, cheaper olive oils or oils of another botanical origin. In 2015, the National Consumers League (NCL) tested 11 different olive oils purchased at various supermarkets and discovered that six of them, despite being labeled “extra virgin,” in actuality did not meet the standards set by the International Olive Council (IOC).
Though the mislabeling of extra virgin olive oil is cause for major health concern, this year, a court in France has sketched an alternative alarm for consumers as they have now banned the use of the world’s most widely used weed killer in its country’s olive groves.
The controversy
Roundup Pro 360, which is the product brand name developed by Monsanto and now owned by the German pharmaceutical company Bayer, has grown to dominate the herbicide market and lists glyphosate as its active ingredient. A French court has ruled that based on scientific studies, Roundup Pro 360 is “a potentially carcinogenic product for humans, suspected of being toxic for human reproduction and for aquatic organisms.”
In 2015, the World Health Organization (WHO) had classified glyphosate as “probably carcinogenic to humans.” Despite this warning, the European Commission had approved a 5-year license renewal for the substance in November 2017. Partial and total bans of glyphosate also have been issued in about a dozen other countries since the release of that statement, including several other members of the European Union, Brazil, Canada and New Zealand.
Bayer (used interchangeably as Monsanto throughout this article) is appealing the French court’s decision, citing studies that prove glyphosate is safe. Bayer is currently facing more than 9,300 lawsuits over the negative health effects of Roundup and related products.
A European Parliament report revealed that the European Commission’s 2017 decision to extend the license for glyphosate was based on text that had been copied and pasted from Monsanto studies and included in an assessment by the European Food Safety Authority (EFSA) that concluded the substance is safe to use.
What one Greek study revealed
The persistence of glyphosate and its primary metabolite AMPA (aminomethylphosphonic acid) was monitored in two areas in Southern Greece with a known history of glyphosate use, and the levels of residues were linked to spray operators’ activities. During a 3-year monitoring study, a total of 170 samples were collected and analyzed from both areas. Differences in the level of residues between areas, as well as sampling sites of the same area, were identified. AMPA persisted longer than the parent compound glyphosate in both areas. To translate: the olives contained residual amounts of Roundup Pro 360.
How widespread is herbicide use?
Weed control in olive groves is needed to prevent weeds from soaking up the moisture the olive trees need to thrive. As a result, herbicide use has become a common practice, especially in Spanish olive groves, and has rapidly increased in the past 20 years. In very mountainous regions, close to 90 percent of the olive groves are in a system using no-tillage and herbicides for weed control.
Why should the American consumer beware?
Olive oil is made by crushing olives into a paste with steel blades. The olives are stirred to release the oil droplets in a process called maceration before being spun in a centrifuge to pull out the oil and water. After the water is removed, what is left is olive oil. If the olives used in this process are contaminated with Roundup Pro, the consumer may be potentially ingesting dangerous cancer-causing (otherwise known as carcinogenic) chemicals.
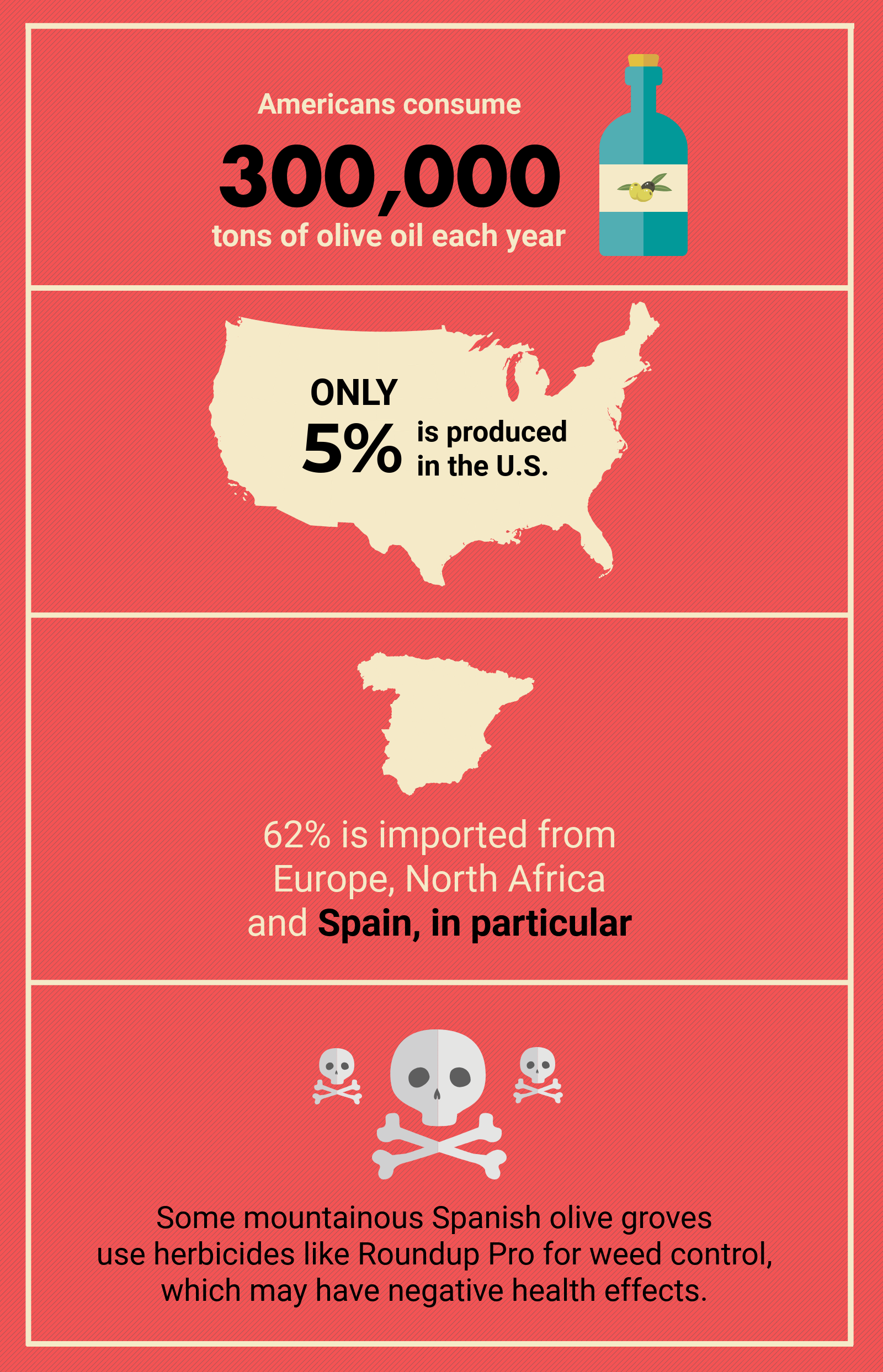
Each year Americans consume more than 300,000 tons of olive oil and less than 5 percent of it is produced in the United States. The bulk of U.S. olive oil imports come from Europe, North Africa, and especially Spain, which accounted for 62 percent of all olive oil imports in 2016.
The EPA’s alleged collusion with Monsanto
Studies conducted by the Environmental Protection Agency, the U.S. agency that regulates the use of herbicides and pesticides, claims the effects of glyphosate dietary exposure contradicts the declaration that it poses carcinogenic hazards by the World Health Organization. This inconsistency in study findings is subject to an ongoing investigation by EPA’s watchdog that is currently investigating allegations that former agency official Jess Rowland colluded with Monsanto during the review process to counter suggestions it endangers human health.
Exactly how much glyphosate is needed to pose the risk of cancer?
The limit in Europe is currently set at 0.5 milligrams (or 500 mcg) of glyphosate daily per kilogram of body weight, which works out to about 34 milligrams, or 34,000 mcg, for a 150-lb. person. The U.S. daily limit, set by the EPA, is 3.5 times as high as Europe’s, although some have called for a lower limit. We think the United States should adopt the stricter standards embraced by Europe.
What is NCL doing?
While regulatory agencies come to a common consensus on the use of Roundup Pro in the cultivation of olives, we at NCL continue to hold olive oil producers accountable for truth in labeling violations. We endorse the use of authentic organic products and believe in its long-term value. NCL aims to monitor product safety and continues to foster the economic protection of the consumer.















