Who We Are
The Preterm Birth Prevention Alliance is a coalition of maternal and women’s health advocates who share a common concern about the state of preterm birth in the United States and the proposed market withdrawal of 17P, the only FDA- approved class of treatments to help prevent spontaneous, recurrent preterm birth. Formed in 2021 by the National Consumers League, we seek to improve preterm birth outcomes in the United States by maintaining access to safe, FDA-approved treatment options and advocating for more diverse medical research that adequately represents the experiences of women and newborns of color. Women of color need a seat at the table.
Our Members
Latest Updates
FDA Announces Hearing Date for 17P
June 14, 2022
The FDA has announced that it expects to hold a long-awaited hearing on the future of 17P on October 17-19, 2022. The virtual hearing will help decide the fate of 17P — the only FDA-approved class of medications that safely reduce the risk of spontaneous, recurrent preterm birth for moms who have already had a preterm baby.
The hearing will be held virtually and follow the agenda available here, on the public docket. The four hours dedicated for public comment over the 2.5-day hearing provides us with a unique opportunity — both as individual organizations and as a collective Alliance. We look forward to amplifying the diverse voices of mothers, babies, families, and advocates as we seek to highlight the need to improve preterm birth outcomes in the United States and address its disproportionate impact on women of color through continued access to FDA-approved treatment options.
We will update this with more information about providing testimony and participating in the hearing as it becomes available.
PBPA Commends HHS Funding to Support Maternal and Infant Health
September 30, 2021
The Preterm Birth Prevention Alliance (PBPA), a coalition of maternal and women’s health advocates dedicated to improving preterm birth outcomes in the United States and addressing its disproportionate impact on women of color…..Read More >
Preterm Birth Prevention Alliance Applauds FDA’s Granting of Hearing for the Only FDA-Approved Therapies to Reduce Recurrent Preterm Birth
August 26, 2021
The Preterm Birth Prevention Alliance, a coalition of maternal and women’s health advocates dedicated to improving preterm birth outcomes in the United States and addressing its disproportionate impact on….Read More >
PBPA meets with the office of Congresswoman Madeleine Dean (PA-4)
July 29, 2021
The PBPA had the opportunity to meet with Representative Dean’s office to discuss the importance of FDA-approved treatment options for women facing preterm birth.
PBPA meets with the White House Domestic Policy Council
July 20, 2021
The PBPA was honored to meet with the White House Domestic Policy Council to discuss maternal and infant health and health equity.
Leading patient advocates launch Preterm Birth Prevention Alliance to protect critical access to the sole FDA-approved class of therapies to reduce recurrent preterm birth
April 20, 2021
Today, the National Consumers League (NCL), along with a coalition of patient advocacy organizations dedicated to advancing the health of mothers and infants, announced the launch of the Preterm Birth Prevention Alliance….Read More >
Alliance Resources
About the Alliance
The Preterm Birth Prevention Alliance (PBPA) formed as a coalition of organizations concerned about the state of preterm birth in the United States and its disproportionate impact on women of color. PBPA’s mission is to improve preterm birth outcomes in the United States by preserving access to safe, Food and Drug Administration (FDA) approved treatment options and advocating for more diverse medical research that adequately represents the experiences of women and newborns of color.
Read More >
Alliance Consensus Statement
The Preterm Birth Prevention Alliance (PBPA) is guided by a core set of principles to advocate for the health interests of at-risk pregnant women and their children. Check out our consensus statement on maintaining access to FDA-approved treatment to prevent spontaneous recurrent preterm birth.
Diverse research for a diverse America: The value of equitable, real-world research
National Consumers League Executive Director, Sally Greenberg, shares her perspective on the important role that expanding diversity and representation in medical research can have on health outcomes, especially maternal health.
Read More >
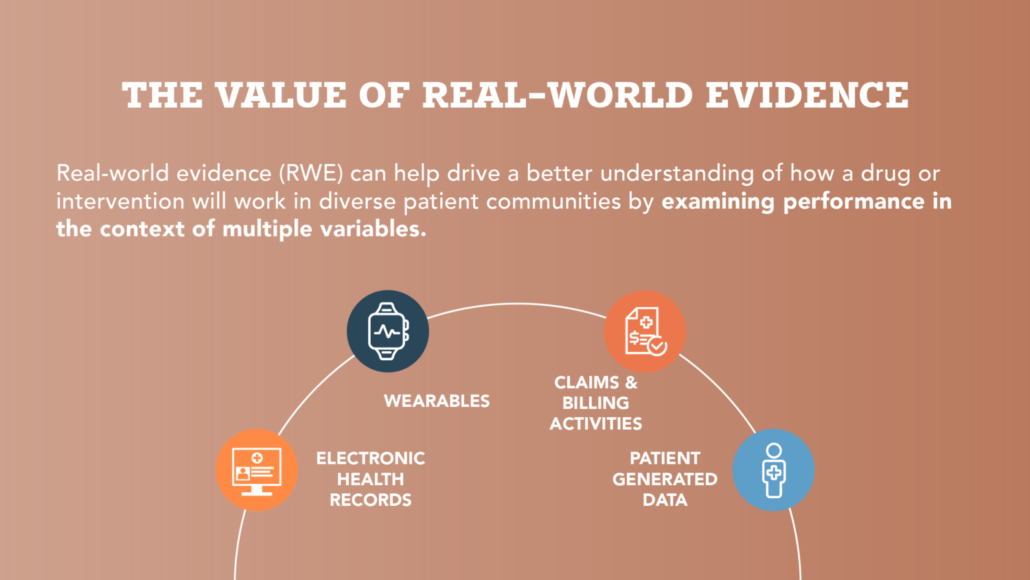
Recognizing the Potential for Real-World Evidence in Maternal Health Infographics
The Preterm Birth Prevention Alliance believes that preterm birth and its disproportionate impact on women of color provide a key example of why we need representative research and Real-World Evidence on treatment efficacy across populations. Check out these infographics for perspectives from recent surveys among women and women’s health, obstetric and neonatal nurses.
Read more on the need for more research across diverse populations >
Read more on women’s perspectives on FDA-approved treatments >
Preterm Birth Resources
Evidence has shown that there may be an increased risk of preterm birth and pregnancy loss among pregnant women with COVID-19. Even aside from COVID risks, premature birth is a leading cause of infant death in the United States. It’s devastating for mothers and families and is very costly to the healthcare system.
Prematurity also has a significantly disproportionate impact on Black, Indigenous, and women of color. According to the March of Dimes, the preterm birth rate among Black American women is 50 percent higher than the rate among all other women.
Only one FDA-approved intervention currently exists to help pregnant women prevent a recurrent preterm birth. It is a prescription medicine called hydroxyprogesterone caproate, which is also known as “17P” or “17-OHPC.” 17P has played a significant role in the treatment of thousands of pregnant mothers and their babies as the only FDA-approved therapy to reduce the risk of recurrent preterm birth and help mothers carry their pregnancies to full-term—which is crucially important to the health of babies.
Recently, the FDA has proposed that 17P be pulled from the market in all forms—branded, generics, and compounded—and advocates are fighting to preserve this resource for women at risk for preterm birth.
Fighting to Protect 17P
Food and Drug Administration Docket
The Food and Drug Administration Docket features perspectives of maternal and infant health advocates, clinicians, and families impacted by prematurity. Statements have been posted by leading stakeholder organizations, including Alliance members such as Black Women’s Health Imperative, HealthyWomen, and Sidelines High-Risk Pregnancy Support Network.
June 23, 2021
Note to CDER Director requesting meeting on preterm birth
The PBPA requested a meeting with Director Dr. Cavazzoni to discuss the public health importance of maintaining access to 17P.
June 21, 2021
PBPA statement urging the agency to protect access to 17P and to hold a public hearing
The PBPA submitted a comment to the FDA docket urging the agency to protect patient access to 17P and to grant a public hearing.
May 11, 2021
Letter to Acting Food and Drug Administration Commissioner requesting meeting on preterm birth
The PBPA requested the opportunity to meet with Acting Director Dr. Woodcock to share the concerns of communities affected by preterm birth.
November 25, 2020
Letter to the Food and Drug Administration from 15 members of Congress
Members of Congress urged the FDA to consider stakeholder voices in its decision surrounding whether to withdraw the only FDA-approved treatment for recurrent preterm birth.
October 14, 2020
The FDA must create a win-win path leading to new data on 17P and protect access for pregnant mothers
National Consumers League Executive Director, Sally Greenberg, published a blog post urging the FDA to factor in the experiences of mothers and healthcare providers in its decision regarding 17P.
October 7, 2020
National Consumers League statement urging FDA to make patient-centered decision on only available treatment option for pregnant mothers at risk for recurrent preterm birth
National Consumers League submitted a comment to the FDA docket urging the agency to center patients in its decision regarding potential withdrawal of 17P.
June 30, 2020
NCL leads advocates in urging FDA to protect patient access to critical therapy for preterm birth
National Consumers League issued a press release highlighting a letter sent to the FDA to protect patient access to 17P.
June 18, 2020
Letter urging FDA to protect patient access to critical therapy for preterm birth
National Consumers League led a group of maternal and infant health stakeholders in sending a letter to the FDA urging the agency to maintain patient access to 17P.
Research and FDA Background
- Role of progestogens in women at risk for spontaneous preterm birth: the final word? (2021)
Ibrahim SA, Haas DM. Lancet;397:1158-1159.
- Safety review of hydroxyprogesterone caproate in women with a history of spontaneous preterm birth (2020)
Sibai B, Saade GR, AF Das AF, Gudeman J. J Perinatol.
- CDER proposes withdrawal of approval for Makena (2020)
U.S. Food and Drug Administration.
- Re-examining the Meis Trial for Evidence of False-Positive Results (2020)
Sibai B, Saade GR, Das AF. Obstet Gynecol;136(3):622-627.
- 17-OHPC to Prevent Recurrent Preterm Birth in Singleton Gestations (PROLONG Study): A Multicenter, International, Randomized Double- Blind Trial (2019)
Blackwell SC et al. J Perinatol;37(2):127-136.
- 4-Year follow-up of children exposed to 17alpha hydroxyprogesterone caproate (17P) in utero (2006)
Northen A. Am J Obstet Gynecol;195(6,56).
- Prevention of Recurrent Preterm Delivery by 17 Alpha-Hydroxyprogesterone Caproate (2003)
Meis PJ et al., N Engl J Med;348:2379-2385.
Contact Us
We’d Like to Hear from You
Please contact us if your organization is interested in learning about our efforts, or sign up to stay up to date on Alliance news and activities.
Lisa McDonald, Vice President of Communications
National Consumers League
lisam@nclnet.org